Developing Sensor Technology
| ✅ Paper Type: Free Essay | ✅ Subject: Technology |
| ✅ Wordcount: 2441 words | ✅ Published: 01 Dec 2017 |
Abstract
The need for sensor devices has been growing to develop new applications in several technological fields. The current state-of-the-art of this sensor technology used in modern electronic nose designs to operate in a different manner. The chamber of the E-Nose sensor is to be upgraded mainly for reducing the nuisance alarms and to improve reliability to detect smoke which is caused by fire and non-fire particles. This paper gives a brief state of the art of different fire and non-fire particles that emits smoke and various chemical gas sensors used to detect smoke and a fire detection algorithm.
Keywords- Sensors; Smoke; Electronic-Noses; Fire Detection Algorithm fire particles; non-fire particles
Introduction
The conception of an electronic nose could appear sort on an up-to-date technology. Scientists initial developed a synthetic nose within the 1930’s that used sensors to measure levels of ultra-violet light found in mercury. Currently these devices are employed in numerous technological fields for various applications.
Presently these devices used as trendy fireplace detection frameworks for the simultaneous estimations of carbon monoxide gas (CO), carbon dioxide (CO2), and smoke. The concentration of the rates of CO and CO2 in smoke offers a path to cut back the frequency of nuisance alarms so as to extend the reliability of smoke detectors. The sensors that square measures incorporated during this fireplace sighting system at the side of fire detection algorithmic rule detect smoke that is caused by fire or non-fire particles, and alarmed accordingly.
Previous fire detection systems used sensors for measuring temperature, smoke, and combustion products which include oxygen (O2), carbon monoxide (CO), carbon dioxide (CO2), water vapor (H2O), hydrogen cyanide (HCN), acetylene (C2H2), and nitric oxide (NO) but they does not give any reliable results. Some used Gas Chromatography – Mass Spectrometry (GC-MS) along with Fourier Transform Infrared (FTIR) Spectroscopy analyzed smoke [1].
Advances in fire detection systems are being sought to decrease the detection time and the frequency of unnecessary alarms. Most of the research works done with the Multi-Sensor Detectors for accomplishing these goals because there may have some trouble in using smoke detectors with a single sensor to discriminate the smoke produced from fire and non-fire sources. The 95% frequency of unnecessary alarms reported by smoke detectors during the 1980’s in the U.S. is due to that limitation.
Section 1 briefly introduces the Fire Detection System incorporated in an Electronic-Nose and different Gas Sensors that detects smoke in Section 2. Later, section 3 gives a brief description about the Fire and Non-Fire Particles and how the sensory system is designed in an E-Nose for preventing Fire accidents in section 4. Finally, we concluded in Section 5.
Chemical Gas Sensors
The environment needs to be monitored [2] time to time as many accidents took place lack of it. So in order to control the Industrial Process, Chemical Sensing Technologies has been emerging out to mainly emphasize on
Control of combustion processes (oxygen)
Flammable gases in order to protect against Fire Explosion.
Toxic gases for environmental monitoring.
Solid Electrolyte Sensor
SE sensor [3] [4] is based on the principle of electrochemical gas detection, which is used to detect chemicals or gases that can be oxidized or reduced in chemical reactions.
It mainly contains three electrodes:
A sensing or working electrode which reacts when gas is available by either oxidizing or reducing the target gas.
A counter electrode which provides a comparing converse response to that occurring at the sensing electrode so as to provide a net current stream.
A reference electrode that stays unaffected by the chemical reactions occurring on the sensing and counter electrodes and provides a stable potential against which measurements are frequently created.

Figure 1. Solid Electrolyte Sensor
SEC sensors (Figure 1) used in millions of vehicles to monitor the exhausted gases and minimize the toxic emissions.
Thermal-Chemical Sensors
Thermal-chemical sensors [2] works on principle that there will be a change in temperature (∆T) when heat energy is released or absorbed (∆Eh). The pellistor is the most common thermal-chemical sensor (other thermal sensors are based on either on thermistors or on thermopiles). They are used for monitoring of combustible gases.

Figure 2.Thermal-Chemical Sensors
Gravimetric Chemical Sensors
They are also known as piezoelectric sensors [5]. They are of two types used for gas sensing – Surface Acoustic Wave (SAW) device and the Quartz Crystal Micro Balance (QCM) as in Figure 4.

Figure 3. SAW Device
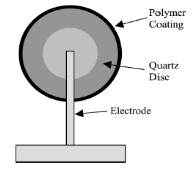
Figure 4. Quartz Crystal Balance
SAW device produces a surface wave that travels along the surface of the sensor while the QCM produces a wave that travels through the bulk of the sensor as shown in Figure 3. Both work on the principle that a change in the mass of piezoelectric sensor coating due to gas absorption results in a change in the resonant frequency of exposure to a vapor.
Conducting Polymer Sensor:
Conducting polymers [2] are plastics and they change their resistance while they adsorb or desorb specific chemicals (Figure 5). The adsorption of these chemicals mainly emphasized on the polarity (charge) and their molecular structure (shape and size).

Figure 5. Conducting Polymer Sensor
Due to their high sensitivity, low price and rapid response time at room temperatures, Conducting Polymer Sensor best suits for chemical sensing.
IR Spectroscopy Sensors:
The Spectroscopic Sensors [2] determine the concentration of several gases at a time and they work on the principle that all the gases interfere and adsorb infrared spectrum at specific wavelengths due to their natural molecular vibration. Some systems with narrow band interference filters or laser light sources for a specific gas (like CO2) are termed as monochromatic systems.

Figure 6. IR Spectroscopy Sensors
In the above Figure 6, some concentration of CO2 present in the sample gas is absorbed by the infrared detector at a wavelength of 4.3 μm while an infrared light periodically emitted from the light source. These sensors are most suitable for CO2 gas and shows low cross-sensitivity with different gasses and are moderate at the reaction, fairly good at accuracy and linearity but are cumbersome and costly.
Optical Fiber Sensors
The optical fiber utilized as a locality of those sensors [6] is coated with fluorescent dye. On association with the vapor, the Polarity variations within the fluorescent dye will changes the dye’s optical properties such as wavelength shift in fluorescence, intensity and spectrum changes. These optical as in Figure 7 changes are used as the retaliation mechanism for gas.

Figure 7. Optical Fiber Sensor
Optical gas sensors are mostly used to detect concentrations of ammonia (NH3). They have very fast response times, short of what 10 micro sec for sampling and analysis and are compact; lightweight can be multiplexed on a single fiber network, immune to electromagnetic interference (EMI) and can operate in high radiation areas.
MOSFET Sensors:
The metal oxide semiconductor field-effect transistor (MOSFET) sensors [4, 7] based on a change of electrostatic potential. They comprise of three layers, they are catalytic metal also called the gate (palladium), a silicon oxide insulator (platinum) and a silicon semiconductor (iridium or rhodium) as in Figure 8. When polar compounds interact with this metal gate, the current flowing through the sensor is modified.

Figure 8. MOSFET Sensor [7]
As no hydrogen atoms are released, molecules such as ammonia or carbon monoxide cannot be detected with a thick metal layer. But it can be possible to detect them when the metal gate is thinned. These MOSFET sensors or MOS sensors are very robust and have a relatively low sensitivity.
E-Nose as Fire Detection System
An electric or artificial nose can sense different types of chemicals and even distinguish particles not only for identifying individuals, but also used for the detection of fire. They work on the principle that smoke is made up of different chemical compounds. These devices consist of dozens of sensors that sense different types of chemical compounds found in the air. Some of the chemicals that cause smoke leads to flames are discussed below.
Smoke
It is a collection of solid and liquid particulates in air and emits gases when a material undergoes combustion or pyrolysis [8]. This is a commonly an unwanted by-product of fires (including candles, stoves, fire ramp and oil lamps), but may also be used for fumigation i.e., pest control. Smoke signals is communication for long distances like smoke signals to transmit signals, news or to indicate the people to gather in a place, offensive and defensive capabilities in the military (smoke-screen), cooking, or smoking like marijuana, tobacco and etc.).
Heptane:
It is a non-polar solvent and minor component of gasoline [9] with chemical formula H3C (CH2)5CH3 or C7H16. This is a colorless liquid and very hazardous chemical that appears which sense like petrolic odor. The structure of Heptane is shown in Figure 9.
|
|
Figure 9. Heptane Structure
It is commercially available as mixed isomers for use in paints and coatings and mainly applied in pharmaceutical manufacturing laboratories and for research & development. It has a melting point at −91.0 to −90.1°C; −131.7 to −130.3°F; 182.2 to 183.0K
Toluene
It is a fragrant hydrocarbon (Its IUPAC deliberate name is methylbenzene) [10] is broadly utilized as a solvent and as an industrial feedstock. It is a water-insoluble clear liquid with the typical smell of paint thinners. In some cases toluene is also used as an inhalant drug for its intoxicating properties; on the other hand, breathing in toluene can possibly cause serious neurological damages.

Figure 10. Toluene Structure
Toluene (Figure 10) is principally utilized as a precursor to benzene. The second positioned application includes its disproportionation to a mixture of benzene and xylene.
Methanol
Methanol is the simplest alcohol, and is a light, unstable, colorless, ignitable fluid with a unique smell as same as to, however marginally sweeter as that of drinking alcohol which we called as ethanol [11]. It is otherwise referred as methyl alcohol, wood alcohol, which is produced as a by-product of the destructive distillation of wood, wood naphtha or wood spirits, with the formula CH3OH for the structure in Figure 11 (often abbreviated MeOH).
|
|
Figure 11. Structure of Methanol
It is likewise utilized for delivering biodiesel by means of transesterification response. At room temperature, it is a polar fluid, and is utilized as a liquid catalyst, dissolvable, fuel, and as a denaturant for ethanol. Methanol is created regularly in the anaerobic metabolism of numerous mixtures of microbes, and is normally present in little sums in the earth.
HDPE Beads
High Density Poly Ethylene Beads [1] are white hermoplastic base resin and looks like wax and have the properties of electric wire.

Figure 12. HDPE Beads
HDPE Beads in the above Figure.12 used for extrusion packaging film, rope, woven bags, fishing nets, water pipes; injection of low-end commodity and housing, non-bearing load components, plastic box, turnover box; extrusion blow moulding containers, hollow products, bottles and it has society of plastic industry resin ID code is 2.
Mixed Plastics
Blended plastic [12] shown in Figure 13 is a term that covers all non-container plastic bundling sourced from the wastage of households, and it incorporates inflexible and adaptable plastic things of different polymer types and shades. It excludes plastic bottles and non-packaging items.

Figure 13. Mixed Plastics
Dry Ice:
Figure 14 shows that it is the strongest manifestation of Carbon dioxide and fundamentally utilized as a cooling agent. It transmutes at −78.5 °c (−109.3 °f) at Earth atmospheric pressures. This great frost makes the strong perilous to handle without protection due to burns caused by freezing (frostbite). It is referred as “Card ice” [13].

Figure 14. Dry Ice
Fire Detection Mechanism in E-Nose
A Novel technique should be employed in E-Nose to respond immediately whenever the fire accidents took place [14]. The main objective of this mechanism is to reduce the nuisance alarms. Several experiments are conducted on various materials that causes smoke and observed how the materials go on burning while ignited them. The table 1 indicates that the ignition method and fire type (how the material burns) of the particular material which causes fire.
Every E-Nose contains a sensory system (two components in E-Nose one is sensory system and the other component is a pattern recognition system [15]) and we need to enhance it so that it can be used as the fire detection system. In the sensory system, one among the above mentioned gas sensors are selected such that they detects particular material’s smoke and according to the classification algorithm and differentiate it whether the smoke is from fire and non-fire particles.
Table 1: List of Particles Causes Fire
|
Sl.No |
Material |
Ignition Method |
Fire Type |
|
1 |
Heptane |
Lighter |
Flaming |
|
2 |
Toluene |
Lighter |
Flaming |
|
3 |
Methanol |
Lighter |
Flaming |
|
4 |
HDPE Beads |
Lighter |
Smouldering |
|
5 |
MixedPlastics |
Coil + Pilot |
Flaming |
|
6 |
Dry Ice |
N/A |
N/A |
The following Figure 15 shows the internal design of sensory system to be deployed in the E-Nose for reducing nuisance alarms as well as to react accordingly to the material that causes a fire.

Figure. 15: Mechanism of Fire Detection System
Based on type of these chemical compounds, the system can give information to the clients about the Fire and Non-Fire particles [16]. The system will perform the perfect action by ringing alert and empowers the fire extinguisher to keep the spreading of kindle to some degree by grouping fire and non-fire particles. The accompanying Table 2 gives the brief description of distinct fire extinguishers feasible in the market.
Table 2: Types of Fire Extinguishers
|
Extinguisher |
Protection Against |
Used For |
|
CO2Fire Extinguisher |
Class B Fires – Petrol,Oil,Paints,Fats |
Online electrical equipment |
|
Water Fire Extinguisher |
Class A Fires – combustible materials like wood and paper |
Common Household purpose |
|
Powder Fire Extinguisher |
Class A,B,C Fires |
General Purpose |
|
Foam Fire Extinguisher |
Class A,B Fires – liquids or materials that liquefy |
Shopping malls |
|
Wet Chemical Extinguishers |
Fires – Cooking Oil or Fats |
Professional Kitchens |
|
Eco Fire Extinguishers |
Fires – Water and Foam |
Environment |
Where each Extinguisher specifies the classes of fires and they are listed below gives the details of their contents for which they belong to.
Table 3: Classes of Fire
|
Class Name |
Contents |
|
Class IA |
Di-ethyl Ether, Ethylene Oxide, Some Light Crude Oils |
|
Class IB |
Motor and Aviation Gasoline, Toluene, Lacquers, Lacquer Thinner |
|
Class IC |
Xylene, Some paints, Some solvent-based Cements |
|
Class II |
Diesel Fuel, Paint Thinner |
|
Class IIIA |
Home Heating Oil |
|
Class IIIB |
Cooking Oils, Lubricating oils, Motor oils |
Conclusion and Future Work
Presently many more fire accidents are taking place and most of them are regarded as nuisance alarms i.e., the sensors that detect smoke will ring the alarm even though it is not necessary. In order to overcome this problem, this paper provided a novel technology that which holds the potential to give numerous benefits in terms of fire accidents like to reduce the nuisance alarms and to increase the reliability of the sensors.
This mechanism not only reduces the false alarms, but also prevents the danger by enabling the in-built extinguisher whenever the fire particle is sensed. In future, we have a tendency to develop the precise classifier algorithm to distinguish the smoke from fire and non-fire particles.
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal






