Comparison of Fuel Types and Suggestions for the Future
| ✅ Paper Type: Free Essay | ✅ Subject: Mechanics |
| ✅ Wordcount: 2578 words | ✅ Published: 18 May 2020 |
Date Submitted:
Throughout the world, many different types of fuels are being used to power vehicles and appliances. All these different types of fuels have certain positive and negative aspects which make them all uniquely different in use. Over the past decade, the need for a sustainable and eco-friendly fuel has grown significantly because of the growing knowledge of global warming and the danger it poses to everyone. In this essay, I am going to explore all the different fuel types and suggest which type would be the most beneficial fuel for the future of Australia.
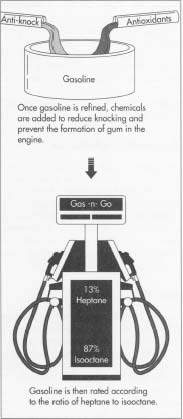
Petrol or gasoline is a colourless liquid derived from petroleum that is primarily used as fuel in spark-ignited internal combustion engines. Petrol is made from crude oil, a substance that is pumped from the ground which consists of decaying plant and animal matter. To make petrol from crude oil there are three necessary steps, fractional distillation, refining petroleum and additives. The first of these step being fractional distillation consists of pumping the crude oil into a furnace and heating it over 316 degrees C causing all the largest molecules to evaporate. This step is necessary as there are large chains of carbon in crude oil, long chains of molecules in crude oil must be separated from the smaller chains of refined fuels, including gasoline to properly refine the crude oil and make petrol. The second stage refining consists of Catalytic cracking. Catalyst cracking is the most important processes in oil refining. This process uses a catalyst at high temperature, an increase of pressure to inflict chemical changes in petroleum. Catalysts such as aluminium, platinum, processed clay, and acids are added to the crude oil to break down bigger molecules so that it will gain the desired compounds of petrol. Another refining process is polymerization. This is the opposite of cracking in that it combines the small molecules of lighter gases into larger ones that can be used as liquid fuels. The final stage, additives consists of improving the quality of petrol, the anti-knock compound is added to the petrol, (In leaded petrol tetraethyl is the anti-knock additive). Other additives (antioxidants) are added to prevent the formulation of ‘gum’ in the engine. Gum is a resin formed in petrol that can be dangerous to the engine inflicting wear if it forms in the engine. Petrol is primarily made up of a mixture of Pure heptane, is a lighter fuel it burns so quickly that it produces a considerable amount of kick to an engine. Pure isooctane makes up about 13% of petrol, it evaporates slowly and unlike heptane produces no kick to the engine. The chemicals are added to improve the quality of the petrol and control the characteristics of it whilst acting as fuel.
Diesel fuel is derived from petroleum, this type of fuel is unique in the way that it does not need a spark to ignite the fuel. Instead, it occurs as a result of compression of the inlet air mixture and then injection of fuel. Diesel is produced by first harvesting crude oil, the result of large decaying quantities of biomass combined with pressure and heat. Once this crude oil has been harvested, it is transported and stored in a refinery. It is here that the oil undergoes three processes to becoming diesel fuel. The first of which is separation then conversion then purification. The separation occurs in large distillation towers, it is here that the oil is exposed to extreme heat, causing it to convert to separate to gasses and liquids. This because of the different boiling points causes the crude oil to separate into three layers, on the top propane gas at the top, diesel is moved to the middle and lubricants near the bottom. The second stage conversion usually consists of applying a catalyst to some of the heavier oils, this catalyst is specially designed to eliminate carbon monoxide, hydrocarbons and particulate matter emission. Modern catalysts consist of a monolith honeycomb structure, packaged in a stainless steel container. The honeycomb structure with many small parallel channels presents a high catalytic contact area to exhaust gasses. As the hot gases contact the catalyst, several exhaust pollutants are converted into harmless substances: carbon dioxide and water. This is the chemical formulas of what actions are occurring through the catalyst.

The 3rd stage and final stage is purification, which usually consists of exposing the products to hydrogen and a catalyst for the removal of sulphur. Although diesel is arguably more fuel-efficient than petrol it has some major drawbacks that make this fuel inefficient in terms of sustainability and the ecosystem. Some pros or advantages of Diesel engines consist of the facts that diesel fuel is highly efficient as it utilizes a compression ignition system that is much more conservative than petrol and gas engines. Diesel cars are much more durable in terms of temperature as they are built to endure the extreme conditions of the energy produced by compression, this causes diesel engines to last longer than gas engines. The cons of diesel fuels mostly consist of environment and economy disadvantages. Firstly both diesel-engined vehicles and diesel fuel itself is very expensive. The fuel although more efficient than petrol is much more expensive. The second con of diesel fuels is the effect on the environment and human life. Diesel emissions contribute to the development of cancer; cardiovascular and respiratory health effects; pollution of air, water, and soil; soiling; reductions in visibility and global climate change. Diesel may be more efficient in terms of efficiency compared to other fossil fuel types, but diesel is still wildly unsustainable and dangerous to the long term survival and habitats of millions of species on earth. This makes diesel a bad choice for a sustainable future of Australia and everywhere else. 
 LPG or liquified petroleum gas or simply propane or butane is a flammable mixture of hydrocarbons gases used as fuel in heating appliances, cooking equipment and vehicles. When LPG is used specifically for vehicles fuel it is often called autogas. LPG, liquefied through pressurisation, comes from natural gas processing, oil refining, drilling oil and gas wells. LPG products are found naturally in combination with other hydrocarbon fuels, typically crude oil and natural gas. LPG is produced during natural gas processing and oil refining. It is isolated, liquefied through pressurisation and stored in pressure vessels.
LPG or liquified petroleum gas or simply propane or butane is a flammable mixture of hydrocarbons gases used as fuel in heating appliances, cooking equipment and vehicles. When LPG is used specifically for vehicles fuel it is often called autogas. LPG, liquefied through pressurisation, comes from natural gas processing, oil refining, drilling oil and gas wells. LPG products are found naturally in combination with other hydrocarbon fuels, typically crude oil and natural gas. LPG is produced during natural gas processing and oil refining. It is isolated, liquefied through pressurisation and stored in pressure vessels.
Biofuels is a type of fuel that has been derived from organic materials such as plant matter and animal matter. The two main types of biofuels manufactured in Australia are bioethanol and biodiesel. To produce bioethanol firstly the milling stage begins, in which the grains are crushed up to release the starch components. Secondly, the crushed grains are heated up, water and enzymes are added to convert the grain mush into fermentable sugar. Thirdly yeast is added and bioethanol is created as the natural product of the sugar fermentation process. The chemical reaction is shown below

The fructose and glucose sugars then react with another enzyme called zymase, which is also contained in the yeast to produce ethanol and carbon dioxide. The chemical reaction is shown below

After the bioethanol is produced it is dehydrated to remove any excess water that would not be beneficial for the engine. Plants capable of undergoing this process include corn, reed canary grass, maize and wheat crops, waste straw, sawdust, willow trees, cord grasses, Jerusalem artichoke, miscanthus and sorghum plants. The ethanol which is produced by the fermentation process still has a high level of water which is necessary to remove. This is done by using the fractional distillation process, the distillation process works by boiling the water and ethanol mixture. Since ethanol has a lower boiling point (78.3C) compared to that of water (100C), the ethanol 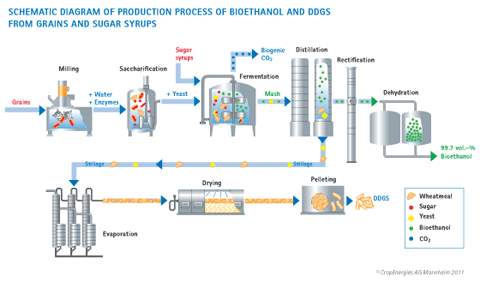
Biodiesel fuel is a sustainable equivalent to diesel, comprised of straight vegetable/ animal oil. Biodiesel is made through a chemical process called transesterification, this is when the vegetal oils or animal fat are converted into methyl ester (biodiesel) and glycerin, a byproduct commonly made into soaps and other products. The biodiesel and glycerin then undergo refining. Both virgin and waste oil (often bought from restaurants) can be used in this process. Biofuels such as ethanol and biodiesel have some major positive aspects but also negative aspects. Below are some of the positive effects biofuels can have because biofuels can be farmed and sourced from local farms and restaurants. Biofuels are a completely sustainable fuel source unlike fossil fuels such as diesel and petrol. Biodiesel typically produces 60% less carbon emission than petroleum-based fuel. Any country can produce biofuels, making it easy for poorer countries to acquire a sustainable and cheap fuel source. Biofuels have the potential to create job opportunities and economic rise. Now for the negative aspects attributed to biofuels, although the fuel itself produces much less carbon dioxide into the atmosphere than fossil fuels. The actual means of production can produce much more carbon emissions than fossil fuels. Biofuels have a lower energy output than fossil fuels, unfortunately making biofuel users buy more fuel creating more carbon emissions. 
Hydrogen gas, although first discovered to be combustible in the 16th century is not widely used today as a truly sustainable and economically friendly fuel. Unlike fossil which has limited supply, hydrogen makes up 75% of the universe, making hydrogen easily available to any manufacturer or supplier. Unfortunately, at the present time, hydrogen gas is too expensive for it to be a success on the public market as its purposes thus far have mostly consisted of large corporate functions such as making ammonia for agricultural fertiliser (the Haber process) manufacturing cyclohexane and methanol, which are intermediates in the production of plastics and pharmaceuticals. Hydrogen gas has also been used in various NASA shuttles as an efficient burner fuel. It is also used to remove sulphur from fuels during the oil-refining process. This steep price could be easily remedied if hydrogen gas became a widespread fuel used in transportation such as cars, buses and motorcycles. The gas itself burns brightly, emitting ultraviolet rays when in an oxygen-rich environment, causing it to be nearly invisible to the naked eye. The gas, when ignited, is highly combustible causing problems for transporting the gas. Hydrogen gas can be extracted from water or hydrocarbons. Today, roughly half the hydrogen produced on the earth comes from natural gas via a steam reforming process. The natural gas reacts with steam in a catalyst converter. The process takes away the hydrogen. 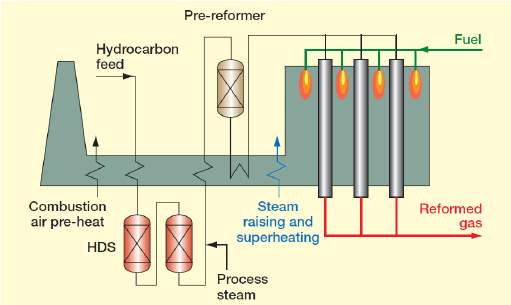
This process, unfortunately, releases carbon dioxide into the atmosphere as a byproduct. There is another way to create hydrogen particles to manufacture into hydrogen gas without using fossil fuels, this is the process of electrolysis. By harnessing the power of wind, geothermal, biomass and hydroelectricity, the electricity can be used in electrolysis to split water into hydrogen and oxygen. The second method of creating hydrogen gas from electrolysis is much cleaner using 100% renewable energy. Hydrogen gas although having many positive aspects about it but at the same time there are certain dangers surrounding it. These are some examples of positive aspects of hydrogen gas. Hydrogen gas is very available, making up 75% of the universe there is no shortage of hydrogen anywhere. While it may take some work to process it there is an infinite abundance in every direction. It is a clean energy fuel, hydrogen gas produces no carbon emissions or harmful byproducts. NASA has even used the water made by the hydrogen fuel on their shuttles as clean drinking water for their astronauts. Hydrogen gas is extremely efficient, for every ml of fuel more energy is delivered. This would, of course, mean more savings for vehicle users. The cons for hydrogen gas consist of the high price, hydrogen gas is currently an impractical fuel to be sold because of the high price associated with freeing the hydrogen from its components. Until research and availability improve enough hydrogen gas won’t be available for the public. Another problem associated with hydrogen gas is transporting it, because of it’s high reactivity and combustibility, moving large amounts of hydrogen gas is dangerous and moving small amounts costs both time and money. The final con for hydrogen gas is storing the gas. Compared to other fuels hydrogen gas is very difficult to store, to keep it from becoming ineffective it must be kept in a liquid state, this means that hydrogen gas must be kept and transported under incredible pressure making it highly inconvenient. 
The main fuel types, petrol, diesel, LPG, biodiesel, ethanol and hydrogen gas all have a range of positives and negative aspects. Australia, especially Tasmania have made quite a commendable amount of effort to support the environment but unfortunately, fossil fuels are still hurting and endangering the wildlife and even the great barrier reef. With this interest in mind, the most promising fuel for Australia’s future would have to be Hydrogen gas. This gas is completely eco-friendly and hugely sustainable, unlike fossil fuels and biofuels this fuel is completely non-toxic and environmentally friendly. The gas does currently have problems transporting and storing the gas but the technology does exist. Trucks with compressing tanks are currently in use transporting the fuel. With some advancements, in this field, the gas could be easily produced, transported and sold without a problem. Hydrogen gas also has the most energy output than any of these fuels, making hydrogen fuel the best fuel for the future of Australia.
References
- Alternativeenergy.procon.org. (2019). How Is Hydrogen Fuel Made? – Alternative Energy – ProCon.org. [online] Available at: https://alternativeenergy.procon.org/view.answers.php?questionID=001266 [Accessed 9 Aug. 2019].
- Ayres, C. (2019). 10 Biggest Pros and Cons of Biofuels. [online] Green Garage. Available at: https://greengarageblog.org/10-biggest-pros-and-cons-of-biofuels [Accessed 8 Aug. 2019].
- Biofuels Association of Australia. (2019). What Are Biofuels? – Biofuels Association of Australia. [online] Available at: http://biofuelsassociation.com.au/biofuels/ [Accessed 9 Aug. 2019].
- Conserve Energy Future. (2019). Pros and Cons of Hydrogen Energy. [online] Available at: https://www.conserve-energy-future.com/pros-and-cons-of-hydrogen-energy.php [Accessed 8 Aug. 2019].
- Cropenergies.com. (2019). Production processes > Bioethanol > CropEnergies AG. [online] Available at: http://www.cropenergies.com/en/Bioethanol/Produktionsverfahren/ [Accessed 7 Aug. 2019].
- Ecotricity.co.uk. (2019). The end of fossil fuels. [online] Available at: https://www.ecotricity.co.uk/our-green-energy/energy-independence/the-end-of-fossil-fuels [Accessed 8 Aug. 2019].
- En.wikipedia.org. (2019). Steam reforming. [online] Available at: https://en.wikipedia.org/wiki/Steam_reforming [Accessed 7 Aug. 2019].
- Esru.strath.ac.uk. (2019). What Is Bioethanol. [online] Available at: http://www.esru.strath.ac.uk/EandE/Web_sites/02-03/biofuels/what_bioethanol.htm [Accessed 9 Aug. 2019].
- Garden, H. and Science, P. (2019). How Gasoline Works. [online] HowStuffWorks. Available at: https://science.howstuffworks.com/gasoline2.htm [Accessed 6 Aug. 2019].
- Hahn, E. (2019). LPG: What is LPG? – LPG Gas – Liquefied Petroleum Gas (Uses – How Made). [online] Elgas.com.au. Available at: https://www.elgas.com.au/blog/492-what-is-lpg-lpg-gas-lp-gas [Accessed 4 Aug. 2019].
- Madehow.com. (2019). How gasoline is made – manufacture, making, used, parts, industry, Raw Materials. [online] Available at: http://www.madehow.com/Volume-2/Gasoline.html [Accessed 9 Aug. 2019].
- Madehow.com. (2019). How gasoline is made – manufacture, making, used, parts, industry, Raw Materials. [online] Available at: http://www.madehow.com/Volume-2/Gasoline.html [Accessed 8 Aug. 2019].
- Nett Technologies. (2019). What Is a Diesel Oxidation Catalyst? | Nett Technologies. [online] Available at: https://www.nettinc.com/information/emissions-faq/what-is-a-diesel-oxidation-catalyst [Accessed 7 Aug. 2019].
- Sciencing. (2019). How Is Diesel Fuel Made?. [online] Available at: https://sciencing.com/diesel-fuel-made-5082571.html [Accessed 7 Aug. 2019].
- TA, L. (2019). Diesel engines: environmental impact and control. – PubMed – NCBI. [online] Ncbi.nlm.nih.gov. Available at: https://www.ncbi.nlm.nih.gov/pubmed/11417675 [Accessed 6 Aug. 2019].
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


