Effects of Changing Concentration of Carbon Dioxide in Sea Water on Marine Life
| ✅ Paper Type: Free Essay | ✅ Subject: Environmental Sciences |
| ✅ Wordcount: 1405 words | ✅ Published: 18 May 2020 |
Claim
Acids are more harmful than bases
Research Question
How does the changing concentration of carbon dioxide in sea water effect marine life?
Rationale
In the recent climate change epidemic, the effects of carbon dioxide have been negative to many ecosystems, this includes the marine system. The changing concentration of harmful acids in the atmosphere has made oceans more acidic and the increasing levels of carbon dioxide has a detrimental effect on the planet’s biodiversity. Due to this, numerous species are finding their habitat modified, resulting in lesser resources for species. For example, organisms with delicate carbonate shells or skeletons, that are weakened by slight changes in the ocean’s acid balance, causes a decline in species. A process that is currently taking place in the oceans is acidification, which is where a substance has a lower pH than normal. Recently, it has been realised that higher atmospheric carbon dioxide warming the planet has come at the cost of changing the ocean’s chemistry. This poses the question, how does the changing concentration of carbon dioxide in sea water effect marine life?
Background
Ocean acidification is the ongoing decrease of Earth’s ocean pH, which is caused by the uptake of carbon dioxide from the atmosphere. When carbon dioxide dissolves in the ocean, carbonic acid is formed. Acids like carbon dioxide increase the concentration of a hydrogen ions. The pH drops when there is a higher concentration of hydrogen ions. Over time, the rapid drop of pH in surface waters gradually mixes into deep water thereby affecting the chemical composition of the seawater. Seawater ranges from basic to alkaline, and ocean acidification involves a shift towards acidic conditions. Due to the quick change in ocean chemistry, marine life does not have time to adapt to the change of pH seawater. This has harmful effects on marine life, impacting chemical communication, reproduction and growth (The Royal Society, 2005). The damage caused by acidification is also cascading through the food chain and in the future, could reduce the amount of food the oceans can produce.
Evidence
When carbon dioxide gas from the air dissolves into sea water it makes it more acidic. The building of skeletons in marine creatures is particularly sensitive to acidity. This higher acidity has a terrible effect on marine life, for example, molluscs find it harder to build shells and tiny animals and coral reefs die.
As mentioned previously, oceans are slightly alkaline, with a pH of 8.06 (Rivai, 2018). Calcium carbonate is a key component of marine shells that binds to hydrogen ions. Calcium carbonate is formed when carbon dioxide reacts with seawater to form carbonic acid, which results in increased hydrogen ions, reducing pH of seawater (Kapoor, 2019). This leads to higher acidity which inhibits marine life shell growth and therefore, impacts reproduction in the organisms.
Research has shown that ocean pH has dropped from 8.2 to 8.1 and is further expected to fall 0.4 pH units by the end of the century if carbon dioxide usage is continued at current rates. This is a significant change as, for example, pH 4 is ten times more acidic than pH 5 and 100 times more acidic than pH 6 (The Ocean Portal Team, 2018).
Figure 1: Graph showing the effect of CO2 on pH levels in the ocean (Coast Adapt, 2017)

1
2
Further evidence is shown in the above graph [1] which illustrates the increasing levels of atmospheric carbon dioxide, leading to decreased pH of the ocean. As seen from the graph, pH levels have reduced significantly from approximately 8.13 to 8.08 since 1990 to 2008. This is due to the increase in greenhouse gases, leading to a greater carbon dioxide absorption into the ocean as indicated in graph 1 (Coast Adapt, 2017).
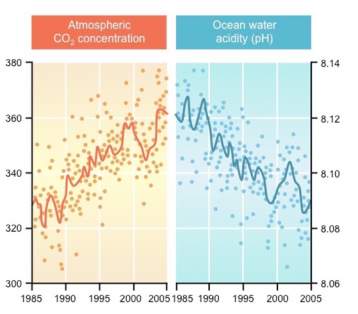 Figure 2: Graph showing the effects of rising CO2 in relation to ocean pH (BioNinja, 2019)
Figure 2: Graph showing the effects of rising CO2 in relation to ocean pH (BioNinja, 2019)
This graph [2] further reiterates the relationship between atmospheric carbon dioxide and ocean acidity data as seen from graph 1 (BioNinja, 2019). It displays how as carbon dioxide levels increase over time, there is an obvious decreasing trend in the ocean’s pH levels.
Due to ocean acidity marine organisms have lowered in abundancy. This is a result from the changes in their growth, development and survival skills. Thus, marine life must learn to survive and adapt as the water around them has an increasing concentration of hydrogen ions. Marine shells are one of the many organisms affected by the decreasing pH of their habitat. This can be seen in the figure below [3] that displays shell deterioration, one of the examples of detrimental effects of ocean acidification.
Figure 3: Images displaying a mollusc shell living under acidic conditions (BioNinja, 2019)

3
This figure [3] displays a mollusc shell dissolving under acidic conditions. After 45 days, the shell almost completely dissolves when placed in seawater with a lower pH and carbonate levels projected by models for the year 2100 (BioNinja, 2019). This is a clear indication of the carbonate shells being broken down by acidic environments, resulting in a change of habitat for marine life.
Similar to molluscs, corals must have access to calcium in the seawater in order to build their hard skeleton. Corals are made up of calcium carbonate and undergo the same process of chemical breakdown due to ocean acidification. This results in coral undergoing adaptations, such as becoming more brittle and resilient to the lowering pH levels in order to survive in the changing marine ecosystem (Dien, Stone, 2018).
Evaluations
The drop in pH in sea water has affected many organisms, however not all have data to see if they corroborate with information found. This is because the ocean is vast and not all organisms have been formally documented or discovered. The research has not taken into account the limitations of undiscovered marine life and the effects of ocean acidification on them.
Different marine species have different homeostasis levels (Biology for Kids, 2018). It has also not been discussed in this report how the effect of lowering pH levels varies between organisms.
The problem with ocean acidification is the sustained nature of the change, as the risk comes from the lifetime exposure to lower pH levels. The rapid pace of acidification will influence the extent to which calcifying organisms will be able to adapt (The Royal Society, 2005).
The information used in this report has come from sources that are relevant and reliable. It is consistent as information can be corroborated with other sources and the facts stated are accepted scientifically. The information is up to date and still considers past data.
Conclusion
It is concluded that the claim has been supported according to this report. The acid researched, carbon dioxide, has been proven to be harmful to the survival of marine organisms and possesses the ability to modify the normally basic conditions of the seawater in the marine ecosystem. This occurs as the dissolved carbon dioxide in the oceans increased hydrogen ions which reduced the pH and caused ocean acidification.
The ability to reduce ocean acidification through artificial methods such as the addition of chemicals is unproven. These techniques will at best be effective only at a very local scale and could also cause damage to the marine environment. A coordinated effort should be launched to monitor the effects of greenhouse gases on the ocean. Models that include the effects of pH at the scale of the organism and the ecosystem are also necessary (The Royal Society, 2005). Reducing carbon dioxide emissions to the atmosphere is one efficient way to minimise the risk of large-scale and long-term changes to the oceans.
Bibliography
- The Ocean Portal Team, (2018). Ocean Acidification. Retrieved August 7, 2019 from https://ocean.si.edu/ocean-life/invertebrates/ocean-acidification
- Dien, K. Stone, D. (2018). The Effects of Ocean Acidification on Coral Reefs. Retrieved August 8 from https://climateinterpreter.org/content/effects-ocean-acidification-coral-reefs
- Biology for Kids, (2018). Regulation – It’s All About Homeostasis. Retrieved August 8 from http://www.biology4kids.com/files/systems_regulation.html
- Kapoor, A. (2019). How does calcium carbonate form in the ocean? Retrieved August 8 from https://www.quora.com/How-does-calcium-carbonate-form-in-the-ocean
- Rivai, Z. (2018). Is sea water alkaline, acidic or neutral? Retrieved August 8 from https://www.quora.com/Is-sea-water-alkaline-acidic-or-neutral
- Coast Adapt. (2017). Ocean acidification and its effects. Retrieved August 6 from https://coastadapt.com.au/ocean-acidification-and-its-effects
- The Royal Society. (2005). Ocean acidification due to increasing atmospheric carbon dioxide. Retrieved August 8 from https://royalsociety.org/~/media/Royal_Society_Content/policy/publications/2005/9634.pdf
- Union of Concerned Scientists. (2011). Ocean Chemistry. Retrieved August 8 from https://www.climatehotmap.org/global-warming-effects/ocean-chemistry.html
- BioNinja. (2019). Ocean Acidification. Retrieved August 8 from https://ib.bioninja.com.au/standard-level/topic-4-ecology/44-climate-change/ocean-acidification.html
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


