Synthesising Benzocaine via Reflux with a Condenser
| ✅ Paper Type: Free Essay | ✅ Subject: Chemistry |
| ✅ Wordcount: 2006 words | ✅ Published: 30 Nov 2017 |
Synthesis and characterisation of benzocaine experiment practical report
Aim: The experiment was to synthesise benzocaine via reflux with a condenser and was characterised by examining at the infra-red spectrum and chemical shifts of NMR.
Introduction: Benzocaine belongs to a collection of medication identified as local anaesthetics and generally consumed as a topical pain reliever. Its mechanism of action is to prevent nerve conduction when applied locally in appropriate concentration to nerve tissues. The main benefit of local anaesthetics is that it does not cause unconsciousness to the patient and its action could be reversible. The use of local anaesthetics presents complete tissue recovery with no permanent damage.
The intention of the experiment was to synthesise benzocaine, an ester, from 4-aminobenzoic acid, a carboxylic acid, by Fischer Esterification. The mechanism in figure 1 was to combined 4-aminobenzoic acid and ethanol in a reflux reaction with the addition of sulphuric acid as a catalyst to produce the product.
 Figure 1
Figure 1
Method: In week one of the experiment, 4-aminobenzoic acid (3.0g), methylated spirits (20cm3) and concentrated sulphuric acid (3.0ml) was added to a dry round bottom flask (100cm3). A condenser was then placed onto the flask. Next, the mixture was placed on a heating mantle and the reflux reaction was carried out for thirty minutes. Afterwards, the reaction mixture was cooled down to room temperature and the condenser was removed. The mixture was gently stirred with a glass rod while sodium hydroxide solution 20 % (20cm3) was added slowly until the mixture has reached neutral using strips of pH paper. Subsequently, pour the contents into a beaker containing 70cm3 of ice and rinse the flask with fresh distilled water into the beaker until the capacity is 150cm3. A Buchner funnel was then used in vacuum filtration to isolate the product. Lastly the product was transferred to a watch glass and dried in the oven for 15 minutes. In week two the experiment was repeated, conversely, isopropyl alcohol was used instead of methylated spirits.
Results:
Percentage Yield = (Actual Yield / Theoretical Yield) x 100
1st week’s percentage yield
C7H7NO2= 12×7 + 7 + 14 + 32= 137g/mol
C2H5OH= 24 + 5 + 16 + 1= 46 g/mol
C9H11NO2= 108 + 11 + 14 + 32 = 165g/mol
1st step: 3g divide 137 g/mol = 0.0219mol  limiting reagent
2nd step: 0.789g/ml of methylated spirit x 20ml (used) = 15.78g
3rd step: 15.78g divide 46g/mol = 0.343mol
4th step: 0.0219mol x 165g/mol = 3.6135g theoretical mass of benzocaine
First week’s product mass (benzocaine): 2.28grams
Percentage yield: 2.28g/3.6135g= 0.63 x 100 = 63%
2nd week’s percentage yield
C7H7NO2= 12×7 + 7 + 14 + 32 = 137g/mol
C2H5OH= 24 + 5 + 16 + 1= 46g/mol
C10H13NO2= 179g/mol
1st step: 3g divide 137g/mol= 0.0219mol  limiting reagent
2nd step: 0.786g/ml of Isopropyl alcohol x 20ml (used) = 15.72g
3rd step: 15.72g divide 46g/mol = 0.342mol
4th step: 0.0219mol x 179g/mol = 3.92g theoretical mass of Isopropyl 4-aminobenzoate
Second week’s product mass (Isopropyl 4-aminobenzoate): 0.29grams
Percentage yield: 0.29g/3.92g= 7.4%
Ethyl 4-aminobenzoate (benzocaine) first week’s product
Experiment melting point: 92-96 degrees Celsius
Reference melting point: 80-90 degrees Celsius
Isopropyl 4-aminobenzoate second week’s product
Experiment melting point: 83-85 degrees Celsius
Reference melting point: 84 degrees Celsius
13Carbon-NMR4-aminobenzoic acid:

|
Carbon |
Chemical Shift(ppm) |
|
1 |
168.0496 |
|
2 |
117.5878 |
|
3 |
131.7728 |
|
4 |
113.1756 |
|
5 |
153.6734 |
|
Solvent DMSO |
40.1326 |
Benzocaine:

|
Carbon |
Chemical shift(ppm) |
|
1 |
14.5155 |
|
2 |
60.3968 |
|
3 |
165 |
|
4 |
120 |
|
5 |
131.6451 |
|
6 |
113.8867 |
|
7 |
150.7141 |
|
Solvent Chloroform |
77.1052 |
H-NMR
4-aminobenzoic acid:

|
Chemical Shift (ppm) |
Splitting Pattern |
Integral Value |
Colour |
|
12 |
singlet |
– |
Light Blue |
|
7.5784, 7.5999 |
doublet |
2 |
Red |
|
6.5367,6.5307,6.5206,6.5092 |
quintet |
5 |
Dark Blue |
|
5.7747 |
singlet |
1 |
Pink |
|
2.4676, 2.4630 |
doublet |
2 |
Solvent DMSO |
Benzocaine:
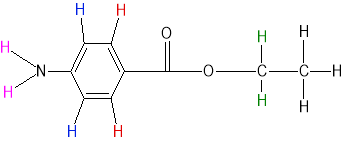
|
Chemical Shift (ppm) |
Splitting Pattern |
Integral Value |
Colour |
|
1.3804,1.3625,1.3447 |
triplet |
3 |
Black |
|
4.3441,4.3264,4.3086,4.2907 |
quartet |
4 |
Green |
|
7.8724,7.8673,7.8556,7.8506 |
doublet |
2 |
Red |
|
6.6550,6.6499,6.6380,6.6330 |
doublet |
2 |
Blue |
|
4.0679 |
singlet |
1 |
Pink |
|
7.2626 |
singlet |
1 |
Solvent (Chloroform) |
Infrared Analysis
(Benzocaine) Week 1 product:
|
Functional group |
Absorption Peak (cm-1) |
|
N-H |
3339.97, 3419.96 |
|
C-H |
3222.91 |
|
C=O and C-H (ester) |
2983.54, 2898.67 |
|
C=O |
1678.70 |
|
C-C (benzene) |
1441.98 |
|
C-N |
1365.61 |
|
C-O (ester) |
1168.85 |
(Isopropyl 4-aminobenzoate) Week 2 product:
|
Functional Group |
Absorption Peak (cm-1) |
|
N-H |
3457.24, 3361.64 |
|
C=O (carbonyl group of ester) |
2824.37 |
|
C-C (benzene ring) |
1441.17 |
|
C-N |
1308.32 |
|
C-O (ester) |
1167.10 |
DEPT-135 NMR
4-aminobenzoic acid
|
Carbon |
Chemical shift (ppm) |
|
either CH or CH3 |
113.1756 |
|
either CH or CH3 |
131.7728 |
Benzocaine
|
Carbon |
Chemical Shift(ppm) |
|
either CH or CH3 |
131.6428 |
|
either CH or CH3 |
113.8944 |
|
CH2 |
60.4045 |
|
either CH or CH3 |
14.5232 |
Discussion of results:
In the experiment, the final appearance of both products appears to be crystalline like powders after drying them in the oven. The first product (benzocaine) melting point appears to be 92-96 degrees Celsius and compare to the theoretical value, it was considered high. Conversely, the second product (isopropyl 4-aminobenzoic acid) melting point was 83-85 degrees Celsius and compare to the theoretical value, it was within the range. Due to the fact that the first product has a higher melting point, it is considered that sample was heated too fast using the heating apparatus or the period the product was in the oven may have been too short therefore
excess water are trapped in the product causing extra hydrogen bonding in the molecule, hence higher energy was needed to break the bonds. Melting point accuracy depicts purity of the product therefore the shorter the range of melting point implies a productive synthesis.
Furthermore, the mass was obtained as the actual yield from benzocaine and isopropyl 4-aminobenzoate to calculate the percentage yield. The theoretical yield of benzocaine was 3.6135grams and the mass obtained in the experiment was 2.28grams, giving a percentage yield of 63 %. This is a high percentage yield, representing a productive synthesis. Conversely, in the second experiment the theoretical yield was 3.92 grams and the mass obtained in the experiment was 0.29grams, giving a percentage yield of 7.4 %. This is a relatively low yield, representing a poor synthesis. This failure synthesis may have been due to several errors including inaccurate instrumental handling techniques, loss of product due to several transferences, inaccurate measurement of resources, and not scraping the entire product out of the Buchner funnel.


(4-aminobenzoic acid) (Benzocaine)
In H-NMR, 4-aminobenzoic acid and benzocaine was identified. By looking at 4-aminobenzoic acid, there were 5 distinct peaks representing 5 distinct proton atmospheres available in the molecule. However the structure of benzocaine, the H-NMR identifies 6 distinct peaks representing 6 distinct proton atmospheres. By observing the peaks in the both molecules, similar singlet peaks were found. The singlet peak for 4-aminobenzoic acid was 5.7747ppm and benzocaine was 4.0609ppm.Both singlet groups were considered amine groups since the 2 hydrogen connected to the nitrogen (pink) creates the same environment therefore only 1 peak was found.
In both molecules, the amine group is considered as an electron donating group since it shields the hydrogen on the adjacent carbons (blue) in the aromatic ring. This shielding causes the hydrogen on the adjacent carbons (blue) to have a lower chemical shift in the spectrum than the hydrogen on the other carbons (red) in the aromatic ring. Furthermore, the hydrogen on the other carbons (red) has a higher chemical shift due to the close distance with the carbonyl group. The carbonyl group is electronegative therefore shifting them at a lower magnetic field.
There is a distinct peak appeared in 4-aminobenzoic acid spectrum, showing a chemical shift of 12 ppm which is considered to be part of the hydrogen of the –COOH (light blue).
As benzocaine has a longer chain compared to 4-aminobenzoic acid, further observation was needed. In benzocaine a peak was found at about 4.2907ppm-4.3441ppm and has a splitting pattern of quartet. A quartet shows that it had 3 adjacent hydrogen atoms therefore it is related to CH2 (green). Furthermore, another peak was found at about 1.3447ppm-1.3804ppm and has a splitting pattern of triplet. A triplet shows that it had 2 adjacent hydrogen atoms therefore it is related to CH3. Since the carbon of CH2 is closer to the oxygen comparing to CH3 therefore it is more de-shielded causing it to be found at a lower magnetic field. Lastly, there were distinct peaks seen in the spectrum for solvent in benzocaine and 4-aminobenzoic acid. In 4-aminobenzoic acid H-NMR, a peak was found at about 2.4630ppm-2.4676ppm and was found to be the solvent DMSO. In benzocaine, a singlet peak was found at 7.2626pm and was found to be the solvent chloroform.
In 13Carbon-NMR, the 4-aminobenzoic acid spectrum has 5 peaks representing 5 distinct carbon atmospheres and benzocaine has 7 peaks representing 7 distinct carbon atmospheres. The peaks are at about 165ppm-168ppm for both molecules represents the occurrence of the carbonyl group (C=O). Furthermore, the solvents were seen on both spectrums. One of 4-aminobenzoic acid’s peaks was at 40.1326ppm which represents DMSO, and benzocaine’s peak was at 77.1052 which represent Chloroform.
In the DEPT-135 NMR (Distortionless Enhancement by Polarisation Transfer), it only exemplifies the occurrence between three kinds of carbon groups, (-CH), (-CH2) and (-CH3). (-CH) and (-CH3) carbons are identified through the positive peaks whereas (-CH2) is identified through the negative peaks on the spectrum. By looking at the 4-aminobenzoic acid spectrum, there are two positive peaks representing those are either (-CH) or (-CH3) carbon groups. In the benzocaine spectrum, the occurrence of (-CH) and (-CH3) carbon groups was identified via the positive peaks. However a negative peak was also identified representing a (-CH2) carbon group has occurred in the spectrum.
The Infrared spectroscopy is useful for analysing the occurrence of different functional groups of the chemical structures such as benzocaine and isopropyl 4-aminobenzoate. By analysing both products, absorption peaks at about 3361cm-1-3457cm-1 on the spectrum were seen at both products. It was considered that the occurrence of the amine group (N-H) was located at the region. Furthermore looking at the structures of both products, the amine group was connected to carbon with the peaks at about 1308.72cm-1-1365.61cm-1 of the spectrum, which represents a C-N bond found in aromatic amine groups. Additionally, looking at the absorption peaks at about 1441.17cm-1-1441.98cm-1 between the two products, it is considered as the aromatic ring (benzene).
Moving through the benzene ring, the spectrum shows the occurrence of the carbonyl group (C=O) that is part of ester group in both products. The absorption peaks measured was about 2824.37cm-1-2983.54cm-1. The carbonyl group of two products were also connected with a C-O bond which is part of the ester group. The peaks measured were 1167.10cm-1-1168.85cm-1 in both products.
Conclusion:
This experiment was considered failure in terms of the weight of the products. It is time-dependent to obtain more accurate yields and handling techniques. Although the melting points were not varied critically, the first product (benzocaine) was not able to achieve a pure product due to high melting point. Although this experiment was considered failure, we still achieved to synthesize 4-aminobenzoic acid to benzocaine and its derivative (isopropyl 4-aminobenzoic acid).
Why do you think the pH of 8 cannot be exceeded in this experiment?
In the experiment, the reactants carboxylic acid (4-aminobenzoic acid) and ethanol were used to synthesize esters (benzocaine, isopropyl 4-aminobenzoic acid) and water molecules as leaving group. The Fischer Esterification reaction mainly functions at pH less than 8. However, increasing the alkalinity atmosphere in this experiment implies the occurrence of additional –OH (hydroxide ions) in the reaction. The additional hydroxide ions could cause the reaction to reverse, making the reactants to be more favourable and fewer products will be form. As mentioned, Fischer Esterification usually functions at pH less than 8. However, increasing the alkalinity environment would cause the reaction saponification to dominate since the reaction is reversed.
How do you expect the Rf value if you have been asked to the synthesize the butyl and pentyl derivatives?
By synthesizing the butyl and pentyl derivatives the Rf value would differ since the polarity with the molecular structure is different. I assumed that the butyl and pentyl derivatives that were made could cause Rf value decreases as the alkyl chain increases. As the chain increases the polarity drops therefore the Rf value decreases.
Reference:
- R. Milnard. The preparation of the local Anesthetic, Benzocaine, by an Esterification Reaction [Internet]. 2006 [updated 2006 October 18; cited 2014 March 7]. Available from: http://courses.chem.psu.edu/chem36/SynFa06Web/Expt86.pdf
- Cerner Multum Inc. Benzocaine Topical [Internet]. 2013 [updated 2013 May 15; cited 2014 March 7]. Available from: http://www.everydayhealth.com/drugs/benzocaine-topical
- Clark Jim. Interpreting C-13 NMR Spectra? [Internet]. 2007 [updated 2007; cited 2014 March 7]. Available from: http://www.chemguide.co.uk/analysis/nmr/interpretc13.html
- Clark Jim. The mechanism for the esterification reaction [Internet]. 2002 [updated 2004; cited 2014 March 7]. Available from: http://www.chemguide.co.uk/physical/catalysis/esterify.html
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


