Polymer Use: Advantages, Disadvantages and Their Respective Impacts on Society
| ✅ Paper Type: Free Essay | ✅ Subject: Chemistry |
| ✅ Wordcount: 2232 words | ✅ Published: 18 May 2020 |
Polymer use advantages and disadvantages and their respective impacts on society
(Science and Society)
Polymers scientifically explained:
What are polymers?
The term “polymer”, ‘poly’ meaning ‘many’ and ‘mer’ meaning ‘repeating unit’ are substances composed of macromolecules (large molecules) commonly used in the making of composites and plastics in its industry (Johnson, 2019). A polymer is defined as a chemical compound which is comprised of long chains of repeating molecular organic subunits called monomers (meaning one individual molecules) (Khan academy, 2019).
(See figure 1 for reaction)
What are the commonly used polymers?
Commonly used natural polymers include proteins (e.g. hair and nails), starches in plants (e.g. maize and potatoes), cellulose (in trees), DNA, wool, silk and rubber (Helmenstine, 2019).
Commonly used synthetic polymers include polypropylene (e.g. upholstery), polyethene low density (e.g. plastic bags), polyethene high density (e.g. detergent bottles), polyvinyl chloride (e.g. piping) and polystyrene (e.g. foam) (Johnson, 2019).
Polymer types and respective properties
Polymer forms can be altered depending on their desired use. Factors that affect their properties include impact resistance, ductility, elasticity, translucence, reflectivity and brittleness. These traits seen in polymers are due to the type of polymerization reaction, such as addition or condensation reactions and how the process is specifically carried out, e.g. altering temperature and pressure (Johnson, 2019). Polymer chains that are tightly twisted result in a stronger physical structure than monomers that are loosely bonded which are more likely to possess flexible and stretchy properties (Johnson, 2019).
Thermoset polymers are altered polymers that are used to increase the strength of a polymer. Cross-linked bonds cannot re-bond due to the presence of strong covalent bonds, leading to rigidity through molding by different levels of heat and pressure (Johnson, 2019).
Thermoplastic polymers are held together by weak intermolecular forces. (University of York, 2013). This gives flexible traits making it possible to change shape through applied heat, e.g. bottles can be melted, reshaped and recycled (Johnson, 2019)
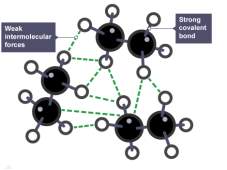

Thermoplastic Thermoset
(BBC Bitesize, 2019) (Karuppiah, 2016)

(“Understanding Plastics and Polymers – The Different Types of Plastic, 2019).
How are they made and the types of polymer reactions to do so
There are two main polymerization processes;
1. Addition/chain reaction
When multiple ethene molecules react with the presence of a catalyst (e.g. Platinum), polyethene is made by the breaking of the (pi) double bonds in which forms the monomers into a chain (Sharpe, 2015).
Chemical Structure:

Catalyst
Ethene
Polyethene
Figure 1.
(where ‘n’ represents number of repeated monomer units)
Chemical Equation:
nH2C=CH2 → [―CH2CH2―]n
An example of this reaction is chloroethene (or vinyl chloride) forming into poly(chloroethene) (PVC) through addition polymerization:
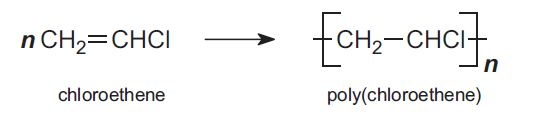

(University of York, 2013)
2. Condensation reaction
Condensation polymerization reactions are different in that instead of double bonds, these monomers have at least two functional groups (alcohol, amine, or carboxylic acid) and these form a linkage chain reaction (Kotz and Treichel, 2019).
The esterification process illustration:

An example of this process is the producing of polyamide 6,6 by using two monomers:
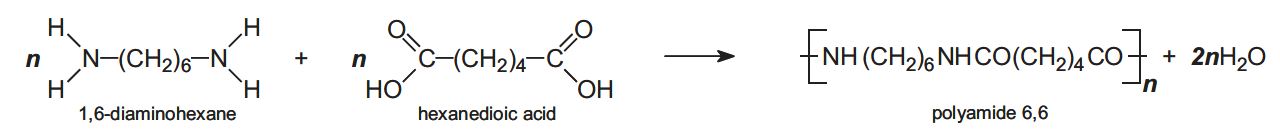

(University of York, 2013)
What is their structure and how does it affect their use?
Polymers are useful due to being capable of altering and tailoring their structures to produce the favored material, which includes a large range of structure, colour and transparent properties (NDT, n.d.).
The types of bonds present (single, double, triple), atom arrangement, geometry and intermolecular forces determines the characteristics of polymers (BBC Bitesize, 2019). This ultimately decides its desired use.
For example, the geometry of the structure affects their packing of molecules, hydrocarbons with 2 bonds (triple bond) are linear and pack better than carbons with 3 bonds (double bond) that are trigonal but flat, carbon with 4 bonds (single bond) are tetrahedral which doesn’t pack as well as 2 and 3 bonds (Kotz and Treichel, 2019).
Table of polymer structure properties:

Polymer impact on society and proposed solutions to those that have become issues
What are the main benefits of plastics in society?
- Versatility, low cost production and the ease of manufacture are just a handful of the benefits of plastics (American Chemistry Council, 2019).
- Infrastructure of buildings, homes and bridges to be durable, sustainable and long-lasting. The main plastics involved: PVC, polyethene and polypropylene (American Chemistry Council, 2019).
- Automotive industry in terms of fuel efficiency, performance and safety. This includes polymers such as polyester (polyethene terephthalate) and nylon fabric (polycaprolactam) which are present in the manufacture of seatbelts and airbags (American Chemistry Council, 2019).
- Greenhouse gas emissions and fuel usage have been reduced with plastics. This is due to the decrease in weight when transporting because of plastic material replacing old material and simultaneously better preserving and protecting sent goods (American Chemistry Council, 2019).
- Plastics in electronics: lightweight, portable, durable and more affordable products such as computers, televisions, cell phones and microwaves. (American Chemistry Council, 2019).
- Sport industry safety gear: protective padding, goggles, mouth guards and helmets. Which include shock-absorbent plastic foam and rugged plastic shells (American Chemistry Council, 2019).
- Medical industry, polymers improve drug bioavailability and the use of artificial skin (IUPAC, 2019).
Which type of polymers can be recycled and what are the respective uses and issues?
Thermoset or thermoplastic category affect the recycling capacity of polymers. No chemical bonding takes place for thermoplastic polymers, therefore allowing reversibility, and as such, the physical properties of thermoplastics are sustained when recycled (Modor Plastics, 2017).
Conversely, thermoset polymers cross-link together which forms irreversible chemical bonds. Consequently, thermosets show efficiency when used for high melting point applications but lack the ability to be successfully recycled (Modor Plastics, 2017).
|
Polymer (thermoplastics) |
Used in/Uses |
Issues |
Societal Solutions (reuse to reduce) |
|
Polyethene terephthalate |
Single use applications: water bottles, cooking oil and packaging. |
Repeated use increases bacterial growth and risk of leaching. Difficult to decontaminate due to harmful chemicals needed to extensively clean. |
Use reusable beverage containers instead. Replace disposable food packaging with reusable alternatives. |
|
High-density polyethene |
Stiff rigid hard-wearing plastic: milk jugs, cleaning agents and waste bins. |
Substances can be contaminated due to contaminants within plastic resins. |
Replace disposable produce bags with reusable alternatives. Low metal content needed. |
|
Low-density polyethene |
Flexible and tough plastic use: squeezable bottles, grocery bags and to package bread. |
Substances can be contaminated due to contaminants within plastic resins. |
Replace plastic grocery bags with fabric alternatives. Or use a reusable cloth bag when shopping. Low metal content needed. |
|
Polypropylene |
Heat/ chemical/moisture resistant and lightweight plastic use: buckets, small containers, rope and packaging tape. |
Generally considered safe, apart from general issues from all plastics such as landfill, toxic additives and leached toxins through incineration. |
Replace with reusable alternatives such as reusable plastic water bottles and straws. |
|
Polystyrene |
Lightweight, malleable, inexpensive plastic use: Styrofoam cups and containers, egg cartons and foam packaging peanuts. |
Vastly dispersed through the environment especially in the sea due to brittle nature, disrupting aquatic life. |
Replace plastic cups, cutlery and containers with reusable stainless steel or compostable/biodegradable substitutes. |
(Table reference: (Seaman, 2012))
In general, most of these different types of polymers come with consequences when recycling such as toxin leaching from additives. This releases toxic fumes into the atmosphere that contributes to global warming (Soffar, 2016).
Which type of polymers are biodegradable? Is that an alternative to recycling?
The branch method of recycling such as incineration requires the burning of polymers which releases harmful compounds such as CO2 and CO and soot that negatively affect the environment and as a result contribute to global warming. On top of that, an extensive amount of energy is consumed within multiple separate factories during the whole process, leaving it to be inefficient (Soffar, 2019). When polymers can no longer be recycled, they are placed in landfills which negatively effects the surrounding environment and blocks space for example agriculture (Soffar, 2019). Especially if the landfill is the sea, which destroys aquatic ecosystems (potential extinction) and the only drinking source for some third world countries.
Some natural biodegradable polymers include starch, pectin and proteins. Some synthetic biodegradable polymers include polycaprolactones, poly(lactic acid) and polyhydroxy-alkanoates (“Biodegradable Polymers and Types of Biodegradable Polymers”, 2017). Biodegradable polymers decompose in between three to six months (Serle, 2019), whereas non-biodegradable polymers takes anywhere from 450-1000 years to decompose (Kari, 2011). The byproducts of biodegradable polymers are eco-friendly; however, the manufacturing of biodegradable materials is currently on average more expensive than the recycling process (Ayres, 2019). Which makes biodegradable polymers currently a better alternative than recycling in removing polymer waste efficiently, reducing the negative environmental impact but more expensive economically.

(“Biodegradable Polymers and Types of Biodegradable Polymers”, 2017).
Are polymers toxic?
The chemical additives and residuals added to polymers are toxic, not the polymers themselves. For example, non-covalent bounded substances such as degradation products in plastic polymers leach out to have harmful effects on the environment (University of Gothenburg, 2011). During the manufacture of plastic, chemical additives are used which carry the toxic aspects of plastic polymers which are leached into society and environmental affecting health negatively (Blake and Rossi, 2014). This toxicity can be avoided by testing whether or not the additives within the given polymer are necessary to its demanded properties. For example, eliminating the need for toxic additives such as flame retardants by redesigning the electronic products to not have plastics in proximity of other parts with high temperatures will reduce the harmful chemical footprint while simultaneously improving consumer products (Blake and Rossi, 2014).
How does micro vs macro plastics affect ecosystems?
Microplastics are defined as particles created when larger plastics classified as macro plastics break down to less than 5mm in diameter before polluting the surrounding environment (National Ocean Service, 2019). The most urgent negatively affected ecosystems are of the sea related ecosystems. There will be less fish than plastic in the earth’s oceans by 2050 if the current rate of unremoved polymer waste continues (Solar Impulse Foundation, 2019). Macro plastics eventually break down from UV radiation from the sun over time into microplastics. The microplastic fragments add to other toxins such as pesticides, hydrocarbons and flame retardants which becomes further concentrated (Beaudry, 2019).
Entanglement and ingestion of these concentrated fragments by marine animals negatively affect their natural reproduction cycle, immunity and all-around survivability of species (Lucas, 2018), such as half the coral cover for the Great Barrier Reef (WWF Australia, 2018). As an unintended consequence, the aquatic animals prematurely die than intended due to the increased accumulated pollutants in their tissues, the natural food chain then becomes disrupted due to the escalation of microplastics traveling up the chain (Lucas, 2018). Another unintended consequence is that microplastics can absorb the water-soluble compounds found in rainwater that in turn creates leachate, which can leach out into groundwater, soil and streams poisoning ecosystems (Bryce, 2015). In terms of society, a large source of food is diminishing slowly and consuming it could transfer the microplastics into our bodies (Lucas, 2018).
Conclusion:
Should plastics be banned?
Polymers are economically efficient due to low cost to manufacture and are efficient in serving to fulfil the demands of society. However, issues arise when it comes to the practice of disposing polymers once their use is lost (“Problems with Polymers”, n.d.). This is usually the reason why landfill around the world and marine plastic pollution continues to increase (Lucas, 2018).
Plastics have many pros and cons. Whilst their disadvantages seemly overpower their usefulness, plastics remain as one of the most influential and useful materials in society. However, plastics are integral to this society, we are very dependent on them and an outright ban on the use of plastics would see many adverse effects on the productivity of our society and would have many economic repercussions. Similarly, the continued use of plastics would lead to ongoing detrimental effects on the environment. A solution to this would include an international effort working towards decreasing the release of harmful byproducts into food, ecosystems and the environment by using more efficient and safe solutions to remove polymer waste such as biodegradable/compostable polymers, reuse alternatives and as a society to be less dependable on harmful plastics.
Reference list:
- https://coggle.it/diagram/WdNWGzONpAABB-qM/t/problems-with-polymers
- https://www.thoughtco.com/what-is-a-polymer-820536
- https://www.khanacademy.org/science/biology/macromolecules/introduction-to-macromolecues/a/introduction-to-macromolecules
- https://www.thoughtco.com/what-are-examples-of-polymers-604299
- https://www.britannica.com/science/polymerization
- http://www.essentialchemicalindustry.org/polymers/polymers-an-overview.html
- https://www.researchgate.net/figure/Molecular-Structure-of-Thermoplastic-and-Thermoset-Polymers-8_fig2_329156276
- https://www.bbc.com/bitesize/guides/z8kgqhv/revision/1
- https://www.azom.com/article.aspx?ArticleID=17477
- https://www.aiche.org/resources/publications/cep/2015/september/making-plastics-monomer-polymer
- https://www.britannica.com/science/chemical-reaction/Polymerization-reactions
- http://www.nde-ed.org/EducationResources/CommunityCollege/Materials/Structure/polymer.htm
- https://www.chemicalsafetyfacts.org/plastics/
- https://iupac.org/polymer-edu/what-are-polymers/
- https://www.modorplastics.com/plastics-learning-center/thermoset-vs-thermoplastics/
- https://learn.eartheasy.com/articles/plastics-by-the-numbers/
- https://www.online-sciences.com/industries/plastic-recycling-advantages-and-disadvantages/
- http://semesters.in/biodegradable-polymers-and-types-of-biodegradable-polymers-notes-pdf-ppt/
- https://connectusfund.org/7-advantages-and-disadvantages-of-biodegradable-plastics
- https://science.gu.se/english/News/News_detail/plastic-products-leach-toxic-substances.cid991256
- https://www.blastic.eu/knowledge-bank/impacts/toxicity-plastics/
- https://www.greenbiz.com/blog/2014/07/01/5-steps-reduce-chemical-footprint-plastic-products
- https://oceanservice.noaa.gov/facts/microplastics.html
- https://solarimpulse.com/plastic-pollution-solutions
- https://www.thoughtco.com/what-are-microplastics-1204133
- https://www.bbc.com/bitesize/guides/z8kgqhv/revision/1
- https://www.wwf.org.au/what-we-do/oceans/great-barrier-reef#gs.svailb
- https://www.sciencefocus.com/science/how-long-do-biodegradable-bags-take-to-decompose/
- https://www.postconsumers.com/2011/10/31/how-long-does-it-take-a-plastic-bottle-to-biodegrade/
- https://ed.ted.com/lessons/what-really-happens-to-the-plastic-you-throw-away-emma-bryce/review_open#question-2
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


