Green Chemistry – Importance and Applications
| ✅ Paper Type: Free Essay | ✅ Subject: Chemistry |
| ✅ Wordcount: 1987 words | ✅ Published: 18 May 2020 |
“Green Chemistry – Importance and Applications”
Green Chemistry also is known as sustainable chemistry looks at chemical products and processes that try to eliminate pollution from the generation of hazardous substances or by-products. Its objective is to reduce pollution by preventing hazardous by-products or substances from being generated.
Green chemistry can have plenty of benefits for one’s health, the economy and environment. Such benefits ae seen from:
Fewer synthetic steps, often means quicker production thereby increasing plant efficiency and water savings.
Cleaner air means there is fewer toxic chemicals being released into the environment.
The use of fewer harmful reagents would result in an increase in the health of staff and analysts in the pharmaceutical industry.
Reducing the usage of dangerous solvents makes food healthier as toxic chemicals can enter the food chain as readily.
As a result of greener chemistry plants and animals in the ecosystem suffer less damage from hazardous chemicals.
Without the use of sustainable chemistry, the potential for global warming will rise, resulting in ozone depletion and smog production.
There are twelve principles to green chemistry, these were introduced by Paul T. Anastas and John C. Warner as “design rules” to help chemists maintain green sustainability for all aspects of the product life cycle.
“1. It is better to prevent waste than to treat or clean up waste after it is formed.
2. Synthetic methods should be designed to maximize the incorporation of all materials used in the process into the final product.
3. Wherever practicable, synthetic methodologies should be designed to use and generate substances that possess little or no toxicity to human health and the environment.
4. Chemical products should be designed to preserve the efficacy of function while reducing toxicity.
5. The use of auxiliary substances (solvents, separation agents, etc.) should be made unnecessary whenever possible and, when used, innocuous.
6. Energy requirements should be recognized for their environmental and economic impacts and should be minimized. Synthetic methods should be conducted at ambient temperature and pressure.
7. A raw material or feedstock should be renewable rather than depleting whenever technically and economically practical.
8. Unnecessary derivatization (blocking group, protection/deprotection, temporary modification of physical/chemical processes) should be avoided whenever possible.
9. Catalytic reagents (as selective as possible) are superior to stoichiometric reagents.
10. Chemical products should be designed so that at the end of their function they do not persist in the environment and instead break down into innocuous degradation products.
11. Analytical methodologies need to be further developed to allow for real-time in-process monitoring and control before the formation of hazardous substances.
12. Substances and the form of a substance used in a chemical process should be chosen so as to minimize the potential for chemical accidents, including releases, explosions, and fires”
(Ahluwalia, V. K. 2013)
12 principles explained with examples:
1. Prevention of waste:
The E-factor (Environmental Impact Factor) was developed by Roger Sheldon to help determine the amount of waste generated per kg of product. The ethylene oxide synthesis reveals how the E-factor does not always consider by-products. For example, the E-Factor for this product should be equal to 5. For every kg of material, 5 kg of waste has been disposed of. Nevertheless, this reaction did not take into account the by-products of chlorine derived from wastewater that was contaminated. As a result, it was necessary to develop a more effective and greener synthesis.
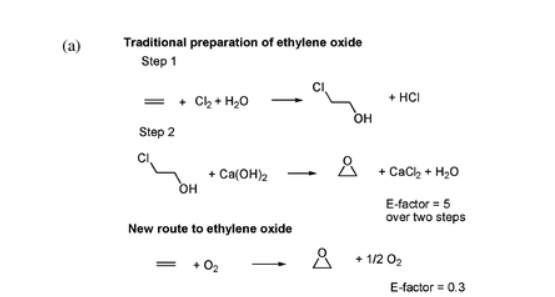
Traditional preparation of ethylene oxide and the new route relying on molecular oxygen.
(P. Anastas & N. Eghbali, 2009)
2. Atom economy (AE):
AE determines how to maximize the use of raw materials in such a way that the final product will have the total number of atoms required to react. The AE formula measures the potential proportional percentage of starting ingredients, which ends up as the necessary and useful reaction products.
Hofmann Elimination reaction, Witting reaction (uses high-mass phosphorus reagents that eventually turn into waste) and Grignard reaction (produces a stoichiometric quantity of phthalic acid) may start with 100% yield, but they don’t consider the large amounts of by-products made.
3. Less Hazardous Chemical Synthesis
Scientists using hazardous chemicals should wear PPE and use respirators. Disposal of hazardous chemicals can be costly, but to maintain safe chemistry is important.
4. Designing Safer Chemicals
Products should have a low toxicity level but still, have able to function as per design. Thalidomide is a key example of a drug that had serious toxic and detrimental effects. This drug was used to treat morning sickness for pregnant women in the 1960s. Unfortunately, Thalidomide was the cause of consequential birth defects in babies born from women receiving this drug, hence the drug was banned and removed from the market. As a result of this incidence, strict regulations were brought into regulation to test all new drugs for their toxicity effects.
5.The Use of Auxiliary Substances
Many solvents are toxic/corrosive and/or flammable. Volatility from such solvents can contribute to air, water and land pollution-reducing green chemistry. Solvent-less systems like H2O, supercritical fluids (SCF) e.g. CO2 and ionic liquids are also exampling new “green” alternatives.
6. Design for Energy Efficiency
Energy required for a reaction should be kept to a minimum. Renewable energy that can be utilized efficiently in science to create energy is Solar energy. Other examples include wind power, hydropower, and geothermal energy.
7. Use of renewable materials
Petroleum oil is an example of a non-renewable source, which comes from petrochemicals.
Starting materials like cellulose, lignin, suberin, and other wood compounds are all renewable energy sources that can come from agricultural products. However, these cannot be gotten in continuous large bulk because of inevitable factors like lack of crop growth due to a shortage of rainfall.
Some other examples of renewable starting materials include carbon dioxide (which can come from both natural and synthetic process) and Methane which can be found from natural and marsh gas).
8. Reduce Derivatives
Where possible, usage of derivatives such as protecting groups should be reduced or avoided. Protecting groups are typically used as they can help avoid changes in certain parts of the structure of a molecule during a chemical reaction while allowing changes to other parts of the structure. This results in an increase in the amount of waste produced, as this reaction requires additional reagents.
9. Catalysts
One major way of improving efforts for greener chemistry is by Switching from stoichiometric processes to catalytic ones. Catalysts lower the energy input required by avoiding the use of stoichiometric reagents.
A typical example is the reduction of a triple bond to double bond by palladium barium sulphate. Different types of catalysts are available. Examples include phase transfer catalysts and biocatalysts.
10. Design for Degradation
Integrating functional groups such as esters or amides which are recognized by ubiquitous enzymes may help the design of environmental degradable products.
Persistent organic pollutants are compounds that do not break down and can remain in the environment. They are typically halogenated compounds, e.g. DDT originally developed as an insecticide, but has become well recognized for its environmental impacts.
11. Pollution Prevention
Improvement of greener methods are required to minimize and prevent the production of hazardous substances in any chemical process. Sensors, monitors and analytical techniques are vital in all product lifecycles to identify potential hazards that could be present in a process. By having such instrumentation in place one can monitor a chemical process for possible hazardous by-products and prevent any probable accident that could occur.
12. Accident Prevention
To prevent accidents from occurring, it is important to identify and assess any possible health and safety hazards. All hazards whether it’s toxicity, physical hazards like explosives or flammability, ought to be addressed to stop accidents from occurring.
Importance of green chemistry
Most solvents are prepared by reactions following several steps that use stoichiometric reagents and chiral auxiliaries. Many of the biologically active compounds from the pharmaceutical industry are highly oxygenated and are derived from some sort of oxidation reaction. Therefore, there is a greater demand for more efficient oxidation catalysts.
In green chemistry, the choice of solvents and additives used to dissolve many of the compounds in a solution is very significant. Solvents are a major source of waste in industrial chemical processing, hence careful selection must be used to increase reaction rates and lower reaction temperatures.
Applications of green chemistry in the pharmaceutical industry:
In 2002, Pfizer developed a new revamped synthesis for sertraline (an API used to treat depression) which illustrated a greener synthetic pathway. HCl was the active ingredient. The new commercial method demonstrated the benefits of pollution control, showing improved safety and handling of products, decreased power and water use which helped double sertraline’s overall product yield.
Sertraline’s original production process began as a three-step sequence; however, this was simplified down to a single-stage production for the new production process. “The new process consists of imine formation of mono-methylamine with a tetralone, followed by reduction of the imine function and in situ resolution of the diastereomeric salts of mandelic acid to provide chiral pure sertraline with a much higher yield and greater selectivity. They also optimized the process by using more benign solvent ethanol for the combined process.”
(US EPA 2002)
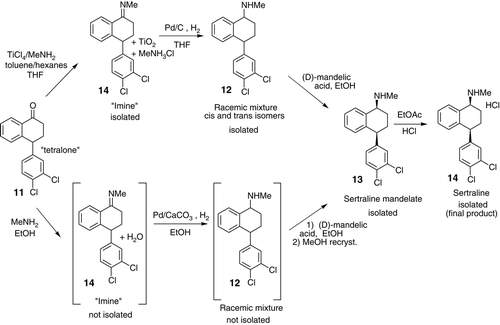
Comparison of routes between the old and new commercial synthesis of Sertraline.
( Ji Zhang 2009)
Pfizer also develops sildenafil citrate (Viagra). Initially, the manufacturing route was not in line with the 12 principles producing low yields giving an overall yield of 4.2% from 2-pentanone. The use of noxious compounds also made this process pathway unsuitable for large scale manufacture. Due to the placement of a highly crystalline, easily purified intermediate in the middle of the synthesis rather than near the end it was also an unsuitable method.

The first preparative route to sildenafil citrate.
Redesign of this synthesis was necessary to increase yield and reduce waste. This was done by placing the cyclization as the last step and utilizing the relatively benign reagents t-butanol and its potassium base and moving chlorosulfonation to an earlier step in the synthesis process. This allowed for purification in the steps before sulfonation which removed the toxic residues. The final step could now also be run in higher concentrations, helping to reduce solvent wastage.

The new synthesis of sildenafil citrate
( Ji Zhang 2009)
Conclusion:
Green Chemistry ensures that all traditional methods of testing and analysis in chemistry is performed in a manner that demonstrates to analysts how non-environmentally friendly solvents and reagent will impact on people and the planet.
Sustainable Chemistry has shown innovation pharma companies can be economically more profitable and more environmentally friendly if utilizing the twelve principles.
While green chemists have done a lot of research to pave the way for a greener future, more work is still needed to sustain a green plant.
|
References |
|
|
|
|
|
|
Cite This Work
To export a reference to this article please select a referencing stye below:
Related Services
View allDMCA / Removal Request
If you are the original writer of this essay and no longer wish to have your work published on UKEssays.com then please click the following link to email our support team:
Request essay removal


