Enthalpy of Combustion
| ✅ Paper Type: Free Essay | ✅ Subject: Chemistry |
| ✅ Wordcount: 1436 words | ✅ Published: 16 Jan 2018 |
- Saran Singh Sound
Aim: To determine the enthalpy change of combustion of Ethanol (C2H5OH), Propanol (C3H7OH) and Butanol (C4H9OH).
Chemicals –
- Ethanol (C2H5OH)
- Propanol (C3H7OH)
- Butanol (C4H9OH)
Data Collection –
Table 1: List of apparatus and Least Count and Uncertainties of Measuring Instruments Used
|
S. No. |
Instrument |
Unit |
Least Count |
Uncertainty |
|
1. |
Digital Stopwatch |
Seconds |
0.01s |
±0.01s |
|
2. |
Measuring Cylinder |
cm3 |
1cm3 |
±0.5cm3 |
|
3. |
Digital Thermometer |
Celsius |
0.1 ºC |
±0.1 ºC |
|
4. |
Digital Weighing Balance |
Grams |
0.001g |
±0.001g |
|
5. |
Cardboard Lid |
— |
— |
— |
|
6. |
Calorimeter |
— |
— |
— |
|
7. |
Spirit Burner |
— |
— |
— |
|
8. |
Wind Shield |
— |
— |
— |
|
9. |
Clamp Stand |
— |
— |
— |
Quantitative Data
- Temperature in degrees Celsius ± 0.1°C
- Time in seconds ± 0.01s
Qualitative Observation:
- It was observed that the flame on the spirit burner swayed due to wind interference. Also, the flame went off at few instances. When the flame was initially burnt its intensity was much greater than when it had burnt for a few seconds.
Section A – Finding the water equivalent of calorimeter
Table 2.2: Collected Data
|
Time/t/s/±0.01s |
Temperature/T/°c/±0.1°c |
||
|
Trial 1 |
Trial 2 |
Trial 3 |
|
|
30.00 |
27.6 |
29.2 |
28.8 |
|
60.00 |
27.6 |
29.2 |
28.8 |
|
90.00 |
27.6 |
29.2 |
28.8 |
|
120.00 |
27.6 |
29.2 |
28.8 |
|
150.00 |
40.3 |
46.0 |
45.3 |
|
180.00 |
39.6 |
45.3 |
44.2 |
|
210.00 |
39.3 |
44.8 |
43.8 |
|
240.00 |
39.0 |
44.4 |
43.4 |
|
270.00 |
38.8 |
44.1 |
43.2 |
|
300.00 |
38.6 |
43.8 |
42.9 |
|
330.00 |
38.3 |
43.6 |
42.7 |
|
360.00 |
38.1 |
43.4 |
42.5 |
|
390.00 |
38.0 |
43.1 |
42.3 |
|
420.00 |
37.9 |
42.9 |
42.0 |
|
450.00 |
37.8 |
42.7 |
41.8 |
|
480.00 |
37.7 |
42.5 |
41.5 |
|
510.00 |
37.3 |
42.3 |
41.3 |
|
540.00 |
37.0 |
42.0 |
41.1 |
|
570.00 |
36.8 |
41.8 |
40.8 |
 Formulas:
Formulas:
Mass = No. of Moles x Molar Mass

Mw x C (Thot – Tfinal) = Mw x C (Tfinal – Tcold) + Q (Tfinal – Tcold)

â-²T = Initial Temperature – Extrapolation Temperature

Enthalpy Change = Mass x Specific Heat Capacity x â-²T

Molar Mass of Ethanol = 46.068g
 Molar Mass of Propanol = 60.095g
Molar Mass of Propanol = 60.095g

Molar Mass of Butanol = 74.122g

Percentage Deviation =  x100
x100
Uncertainty Formulas:

Error in mass of Water =  x 100
x 100

Error in temperature =  x 100
x 100

Error in time =  x 100
x 100

Avg. Uncertainty = 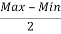
Trial 1:
|
Temperature of cold water/Tc |
27.6 ± 0.1 ºC |
|
Temperature of hot water/Th |
57.1 ± 0.1 ºC |
|
Mass of Water in Clorimeter/Mc |
50.0 ± 0.5g |
|
Specific Heat Capacity |
4.18 J.g‑1.ºC-1 |
|
Initial Temperature |
27.6 ± 0.1ºC |
|
Extrapolation temperature/Tf |
40.4 ± 0.1ºC |
|
Time at which Hot water was added |
120.00 ± 0.01s |
Uncertainty of temperature is small and hence error bars cannot be seen on the graph
The graph is used to estimate the water equivalent of calorimeter. The gradient of the best fit line shows the rate of decrease of temperature.
Uncertainties:-
Error in mass of Water =  x 100 = 1%
x 100 = 1%
Error in Temperature of cold water/Tc =  x 100 = 0.4%
x 100 = 0.4%
Error in Temperature of hot water/Th =  x 100 = 0.2%
x 100 = 0.2%
Error in Extrapolated Temperature/Tf =  x 100 = 0.2%
x 100 = 0.2%
Calculation:-
Mw x C (Thot – Tfinal) = Mw x C (Tfinal – Tcold) + Q (Tfinal – Tcold)
... 50.0 x 4.18 (57.1 – 40.4) = 50.0 x 4.18 (40.4 – 27.6) + Q (40.4 – 27.6)
So, Q = 63.8g
Percentage Uncertainty for Q= 1% + (0.2% – 0.2%) = 1% + (0.2% -0.4%) + (0.2% – 0.4%)
... 1% + 0.6% = 1.6% Uncertainty
Absolute Uncertainty = 1.6% x 63.8 = ± 1g
So, Water equivalent of calorimeter = (64 ± 1)g
Note : There were three assumptions made during this experiment.
- The Specific Heat Cpacity of the calorimeter is same as water
- No Heat is lost to the surrounding
- Mass of water is equal to volume of water
Trial 2:
|
Temperature of cold water/Tc |
30.1 ± 0.1 ºC |
|
Temperature of hot water/Th |
63.6 ± 0.1 ºC |
|
Mass of Water in Clorimeter/Mc |
50.0 ± 0.5g |
|
Specific Heat Capacity |
4.18 J.g‑1.ºC-1 |
|
Initial Temperature |
30.1 ± 0.1ºC |
|
Extrapolation temperature/Tf |
45.6 ± 0.1ºC |
|
Time at which Hot water was added |
120.00 ± 0.01s |


Uncertainty of temperature is small and hence error bars cannot be seen on the graph
The graph is used to estimate the water equivalent of calorimeter. The gradient of the best fit line shows the rate of decrease of temperature.
Uncertainties:-
Error in mass of Water =  x 100 = 1%
x 100 = 1%
Error in Temperature of cold water/Tc =  x 100 = 0.3%
x 100 = 0.3%
Error in Temperature of hot water/Th =  x 100 = 0.2%
x 100 = 0.2%
Error in Extrapolated Temperature/Tf =  x 100 = 0.2%
x 100 = 0.2%
Calculation:-
Mw x C (Thot – Tfinal) = Mw x C (Tfinal – Tcold) + Q (Tfinal – Tcold)
... 50.0 x 4.18 (63.6 – 45.6) = 50.0 x 4.18 (45.6 – 30.1) + Q (45.6 – 30.1)
So, Q = 33.7g
Percentage Uncertainty for Q= 1% + (0.2% – 0.2%) = 1% + (0.2% – 0.3%) + (0.2% – 0.3%)
... 1% + 0.8% = 1.8% Uncertainty
Absolute Uncertainty = 1.8% x 33.7 = ± 0.6 g
So, Water equivalent of calorimeter = (33.7 ± 0.6)g
Note : There were three assumptions made during this experiment.
- The Specific Heat Cpacity of the calorimeter is same as water
- No Heat is lost to the surrounding
- Mass of water is equal to volume of water
Trial 3:
|
Temperature of cold water/Tc |
29.8 ± 0.1 ºC |
|
Temperature of hot water/Th |
62.6 ± 0.1 ºC |
|
Mass of Water in Clorimeter/Mc |
50.0 ± 0.5g |
|
Specific Heat Capacity |
4.18 J.g‑1.ºC-1 |
|
Initial Temperature |
29.8 ± 0.1ºC |
|
Extrapolation temperature/Tf |
44.6 ± 0.1ºC |
|
Time at which Hot water was added |
120.00 ± 0.01s |
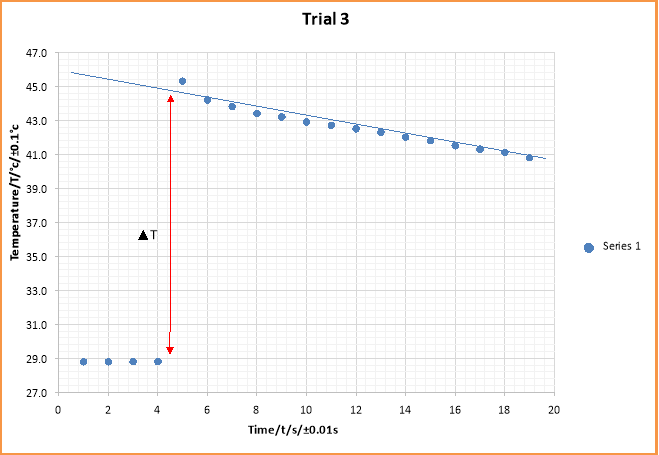
Uncertainty of temperature is small and hence error bars cannot be seen on the graph
The graph is used to estimate the change in temperature of the displacement reaction between CuSO4 solution and Zinc (s) powder. The gradient of the best fit line shows the rate of decrease of temperature.
Uncertainties:-
Error in mass of Water =  x 100 = 1%
x 100 = 1%
Error in Temperature of cold water/Tc =  x 100 = 0.3%
x 100 = 0.3%
Error in Temperature of hot water/Th =  x 100 = 0.2%
x 100 = 0.2%
Error in Extrapolated Temperature/Tf =  x 100 = 0.2%
x 100 = 0.2%
Calculation:-
Mw x C (Thot – Tfinal) = Mw x C (Tfinal – Tcold) + Q (Tfinal – Tcold)
... 50.0 x 4.18 (62.6 – 44.6) = 50.0 x 4.18 (44.6 – 29.8) + Q (44.6 – 29.8)
So, Q = 45.2g
Percentage Uncertainty for Q= 1% + (0.2% – 0.2%) = 1% + (0.2% – 0.3%) + (0.2% – 0.3%)
... 1% + 0.8% = 1.8% Uncertainty
Absolute Uncertainty = 1.8% x 45.2 = ± 0.8 g
So, Water equivalent of calorimeter = (45.2 ± 0.8)g
Note : There were three assumptions made during this experiment.
- The Specific Heat Cpacity of the calorimeter is same as water
- No Heat is lost to the surrounding
- Mass of water is equal to volume of water
Average of all Trials:
Average Water Equivalent of calorimeter/Q =  = 47.6 g
= 47.6 g
Uncertainty for Average Water Equivalent of calorimeter/Q =  = ± 15g
= ± 15g
Therefore, Average Water Equivalent of calorimeter/Q = (48 ± 15)g
Section B – Finding the enthalpy of combustion of three alcohols.
Table 1.1: Collected Data
|
Alcohols |
Trial |
Initial Mass of Spirit Lamp + Alcohol/Mi/g/±0.001g |
Final Mass of Spirit Lamp + Alcohol/Mf/g/±0.001g |
Initial Temperature of water/Ti/°C/±0.1°C |
Final Temperature of water/Ti/°C/±0.1°C |
|
Ethanol |
1. |
136.832 |
136.269 |
28.9 |
46.1 |
|
2. |
136.269 |
135.934 |
29.0 |
47.7 |
|
|
3. |
135.934 |
135.558 |
29.2 |
50.9 |
|
|
Propanol |
1. |
138.554 |
138.087 |
29.2 |
44.9 |
|
2. |
138.087 |
137.812 |
29.0 |
45.9 |
|
|
3. |
137.812 |
137.547 |
29.0 |
46.9 |
|
|
Butanol |
1. |
137.844 |
137.667 |
28.9 |
38.9 |
|
2. |
137.667 |
137.367 |
29.4 |
45.2 |
|
|
3. |
137.367 |
137.208 |
29.6 |
42.4 |
Note: Mass of Water was kept constant at 50.0 ± 0.5g
Enthalpy of combustion (ΔHØC) of ethanol :
Trial 1:
Mass of Ethanol lost = 136.832 – 136.269 = (0.563 ± 0.002)g
Temperature Change/ΔT = 28.9 – 46.1 = (-17.2 ± 0.2)°C
No of moles =  = (0.0100 ± 0.0004)mol
= (0.0100 ± 0.0004)mol
ΔHØC =  = -442040 J.mol-1 =
= -442040 J.mol-1 =  = -442.040 kJ.mol-1
= -442.040 kJ.mol-1
Uncertainties:
Error in mass of Water =  x 100 = 1%
x 100 = 1%
Error in No of Moles =  = 4%
= 4%
Error in Temperature Change/ ΔT =  = 1.2%
= 1.2%
Error in Water equivalent of Calorimeter/Q =  = 31.3%
= 31.3%
Percentage Uncertainty in ΔHØC = 2.2% + 32.5%) + 4% = 38.7%
Absolute Uncertainty = 38.7% x -442.040 = ± 171 kJ.mol-1
So, Enthalpy of Combustion of ethanol = (-442 ± 171 ) kJ.mol-1
Trial 2:
Mass of Ethanol lost = 136.269 – 135.934 = (0.335 ± 0.002)g
Temperature Change/ΔT = 28.9 – 47.7 = (-18.7 ± 0.2)°C
No of moles =  = (0.0100 ± 0.0004)mol
= (0.0100 ± 0.0004)mol
ΔHØC =  = -480590 J.mol-1 =
= -480590 J.mol-1 =  = -480.590 kJ.mol-1
= -480.590 kJ.mol-1
Uncertainties:
Error in mass of Water =  x 100 = 1%
x 100 = 1%
Error in No of Moles =  = 4%
= 4%
Error in Temperature Change/ ΔT =  = 1.2%
= 1.2%
Error in Water equivalent of Calorimeter/Q =  = 31.3%
= 31.3%
Percentage Uncertainty in ΔHØC = 2.2% + 32.5% + 4% = 38.7%
Absolute Uncertainty = 38.7% x -480.590 = ± 186 kJ.mol-1
So, Enthalpy of Combustion of ethanol = (-481 ± 186) kJ.mol-1
Trial 3:
Mass of Ethanol lost = 135.934 – 135.558 = (0.376 ± 0.002)g
Temperature Change/ΔT = 28.9 – 50.9 = (-21.7 ± 0.2)°C
No of moles =  = (0.0100 ± 0.0004) mol
= (0.0100 ± 0.0004) mol
ΔHØC =  = -557690 J.mol-1 =
= -557690 J.mol-1 =  = -557.690 kJ.mol-1
= -557.690 kJ.mol-1
Uncertainties:
Error in mass of Water =  x 100 = 1%
x 100 = 1%
Error in No of Moles =  = 4%
= 4%
Error in Temperature Change/ ΔT =  = 1%
= 1%
Error in Water equivalent of Calorimeter/Q =  = 31.3%
= 31.3%
Percentage Uncertainty in ΔHØC = 2% + 32.3% + 4% = 38.3%
Absolute Uncertainty = 38.3% x -557.690 = ± 214 kJ.mol-1
So, Enthalpy of Combustion of ethanol = (-558 ± 214) kJ.mol-1
Average of all Trials:
Average Enthalpy of Combustion of ethanol =  = -494 kJ.mol-1
= -494 kJ.mol-1
Uncertainty in Average Enthalpy of Combustion of ethanol =  = ± 58 kJ.mol-1
= ± 58 kJ.mol-1
So, Average Enthalpy of Combustion of ethanol = (-494 ± 58) kJ.mol-1
Literature Value = -1368 kJ.mol -1
Therefore, Percentage Deviation = 63.9%
Enthalpy of combustion (ΔHØC) of propanol :
Trial 1:
Mass of Propanol lost = 138.554 – 138.087 = (0.467 ± 0.002)g
Temperature Change/ΔT = 28.9 – 44.9 = (-15.7 ± 0.2)°C
No of moles =  = (0.0100 ± 0.0004) mol
= (0.0100 ± 0.0004) mol
ΔHØC =  = -403490 J.mol-1 =
= -403490 J.mol-1 =  = -403.490 kJ.mol-1
= -403.490 kJ.mol-1
Uncertainties:
Error in mass of Water =  x 100 = 1%
x 100 = 1%
Error in No of Moles =  = 4%
= 4%
Error in Temperature Change/ ΔT =  = 1.2%
= 1.2%
Error in Water equivalent of Calorimeter/Q =  = 31.3%
= 31.3%
Percentage Uncertainty in ΔHØC = 2.2% + 32.5% + 4% = 38.7%
Absolute Uncertainty = 38.7% x -403.490 = ± 156 kJ.mol-1
So, Enthalpy of Combustion of propanol = (-403 ± 156) kJ.mol-1
Trial 2:
Mass of Propanol lost = 138.087 – 137.812 = (0.275 ± 0.002)g
Temperature Change/ΔT = 28.9 – 45.9 = (-16.9 ± 0.2)°C
No of moles =  = (0.0100 ± 0.0004)mol
= (0.0100 ± 0.0004)mol
ΔHØC =  = -434330 J.mol-1 = =
= -434330 J.mol-1 = =  = -434.330 kJ.mol-1
= -434.330 kJ.mol-1
Uncertainties:
Error in mass of Water =  x 100 = 1%
x 100 = 1%
Error in No of Moles =  = 4%
= 4%
Error in Temperature Change/ ΔT =  = 1.2%
= 1.2%
Error in Water equivalent of Calorimeter/Q =  = 31.3%
= 31.3%
Percentage Uncertainty in ΔHØC = 2.2% + 32.5% + 4% = 38.7%
Absolute Uncertainty = 38.7% x -434.330 = ± 168 kJ.mol-1
So, Enthalpy of Combustion of propanol = (-434 ± 168) kJ.mol-1
Trial 3:
Mass of Propanol lost = 137.812 – 137.547 = (0.265 ± 0.002)g
Temperature Change/ΔT = 28.9 – 46.9 = (-17.9 ± 0.2)°C
No of moles =  = (0.0100 ± 0.0004)mol
= (0.0100 ± 0.0004)mol
ΔHØC =  = -460030 J.mol-1 =
= -460030 J.mol-1 =  = -460.030 kJ.mol-1
= -460.030 kJ.mol-1
Uncertainties:
Error in mass of Water =  x 100 = 1%
x 100 = 1%
Error in No of Moles =  = 4%
= 4%
Error in Temperature Change/ ΔT =  = 1.2%
= 1.2%
Error in Water equivalent of Calorimeter/Q =  = 31.3%
= 31.3%
Percentage Uncertainty in ΔHØC = 2.2% + 32.5% + 4% = 38.7%
Absolute Uncertainty = 38.7% x -460.030 = ± 178 kJ.mol-1
So, Enthalpy of Combustion of propanol = (-460 ± 178) kJ.mol-1
Average of all Trials:
Average Enthalpy of Combustion of propanol =  = -432 kJ.mol-1
= -432 kJ.mol-1
Uncertainty in Average Enthalpy of Combustion of propanol =  = ± 29 kJ.mol-1
= ± 29 kJ.mol-1
So, Average Enthalpy of Combustion of propanol = (-432 ± 29) kJ.mol-1
Literature Value = -2021 kJ.mol -1
Therefore, Percentage Deviation = 78.5%
Enthalpy of combustion (ΔHØC) of butanol :
Trial 1:
Mass of butanol lost = 137.844- 137.667 = (0.177 ± 0.002)g
Temperature Change/ΔT = 28.9 – 38.9 = (-10.0 ± 0.2)°C
No of moles =  = (0.0020 ± 0.0004)mol
= (0.0020 ± 0.0004)mol
ΔHØC =  = -1285000 J.mol-1 =
= -1285000 J.mol-1 =  = -1285.000 kJ.mol-1
= -1285.000 kJ.mol-1
Uncertainties:
Error in mass of Water =  x 100 = 1%
x 100 = 1%
Error in No of Moles =  = 0.2%
= 0.2%
Error in Temperature Change/ ΔT = 




